Determine Which Ionic Compound Is Dissolved in Solution.
Specifically we are going to work with ionic compounds that are soluble in water dissocia. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts
Who are the experts.
. Chemistry The following compounds are dissolved in diethyl ether and then extracted with 01 M HCI pH 1 which will be in the aqueous layer. The opposite is a dilute solution. First a sample of the unknown substance can be placed in a test tube and put over a flame.
As summarized in Figure 171 The Relationship between there are three possible conditions for an aqueous solution of an ionic solid. Ionic compounds dissolve in polar solvents especially water. For example consider the following compounds.
In this episode of Real Chemistry you will learn how to determine if an ionic compound is soluble or insoluble. In our example we need to rewrite 7110 9 Pb 2 I - 2 Since there is 1 lead ion Pb 2 in the compound the number of dissolved compound molecules will be equal to the number of free lead ions. When the ions are in solution they are surrounded by water molecules and the ions are said to be solvated or dissolved in an aqueous solution denoted aq.
An equation can still be written that simply shows the solid going into solution. NaCl BaSO 4 NaC 2 H 3 O 2 and CaS. The negative ends of the H2O molecules face the positive ions and the positive ends face the negative ions.
Once we know the compound we use the Solubility Table to determine its solubility. With ionic compounds chemists often talk about ionic concentrations or the concentrations of the individual ions. This process represents a physical change known as dissociation.
Although many ionic compounds dissolve in. Predict whether aqueous solutions of the following substances are acidic basic or neutral. I know for these problems you can sometimes ignore parts of the solution for some reason.
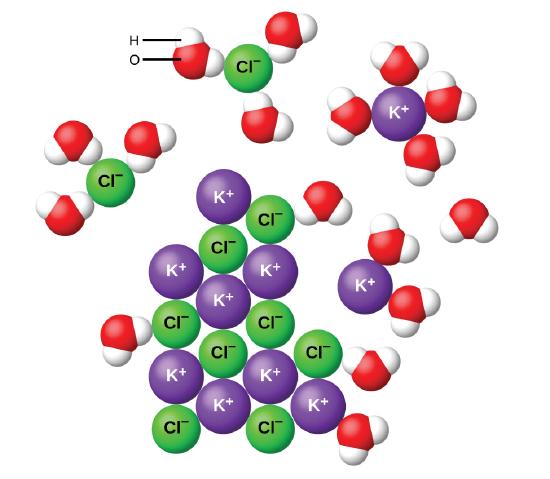
1 Consider the diagram of an aqueous solution of a soluble ionic compound. Ionic compounds dissociate completely when dissolved. If the substance melts then it is probably a covalent simple molecular substance as shown in the table.
This solution can accept more solute. Answer to Solved Consider the diagram of an aqueous solution of a. Consider the diagram of an aqueous solution of a soluble ionic compound determine which ionic compound is dissolved in solution.
Set x equal to the amount of the compound that will dissolve and rewrite the variables representing the numbers of each ion in terms of x. Under most conditions ionic compounds will dissociate nearly. Write the chemical equation that represents the dissociation of each ionic compound.
C 12 H 22 O 11 s C 12 H 22 O 11 aq Summary Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. The chemical formula shows that each formula unit of CaCl2 contains one Ca2 and two Cl ions. When CaCl2 a water soluble ionic compound dissolves in water it splits into its component ions Ca2 and Cl.
This occurs when the positive cation from the ionic solid is attracted to the negative end of the water molecule oxygen and the negative anion of the ionic solid is attracted. This is done to determine the melting point. This means that when one mole of salt NaCl is dissolved in water the resulting solution does not contain any molecules of NaCl it contains the ions Na and Cl -.
In order for NaCl to be soluble the Na and Cl- ions must break free from the crystal-lattice structure of the solid. The solution is saturated and at equilibrium. Nonionic compounds do not dissociate in water.
There are nine 1 positivelly charged ba. This page discusses the solubility of compounds in water at room temperature and standard pressure. Determine the solubility in water.
Determine which ionic compound is dissolved in solution. Experts are tested by Chegg as specialists in their subject area. KBr Na 2 SO 4 Solution KBr s K aq Br aq Not only do the two sodium ions go their own way but the sulfate ion stays together as the sulfate ion.
Determine which ionic compound is dissolved in solution. The dissolving equation is Na 2 SO 4 s 2Na aq SO 4 2 aq Exercise 811. This question has me stumped.
Chemistry questions and answers. LiI K3PO4 CH3NH3Cl I found the portions of my text that deal with this problem but it doesnt seem to go into enough detail. NH2 NH2 OH OH II IV V O l and IV O I II and II O I IV and v I and V O Il and III O II III and IV.
When ionic compounds dissolve in water the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions reducing the strong electrostatic forces between them. Pressure and temperature affect solubility. 3 3 O Na PO MgBrz Naci O AICI.
Consider the diagram of an aqueous solution of a soluble ionic compound 3 Determine which ionic compound is dissolved in solution. The solution is unsaturated and more of the ionic solid if available will dissolve. I understand that the first.
A saturated solution is one in which the maximum amount of solute has been dissolved. However if no melting occurs the substance can either be ionic covalent giant atomic or metallic. When an ionic compound dissolves in water H2O molecules separate surround and disperse the ions into the liquid.
In this video we will go through how to find ions in a compound. For the dissolving of sucrose. Hence the process of dissolving a NaCl crystal can be described by the following chemical equation.
We review their content and use your feedback to keep the quality high. We can use the Solubility Table to determine whether an ionic compound exist as ions in aqueous solution soluble or as a solid insoluble. Consider the diagram of an aqueous solution of a soluble ionic compound.
What Happens To Ionic And Covalent Compounds When They Dissolve In Water Quora

Aleks Calculating The Solubility Of An Ionic Compound When A Common Ion Is Present Youtube

How Do Aqueous Solutions Of Ionic Molecular Compounds Differ Study Com

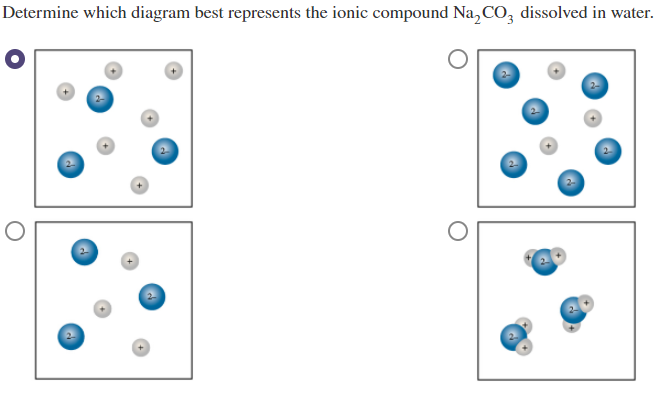
Solved Determine Which Diagram Best Represents The Ionic Chegg Com
No comments for "Determine Which Ionic Compound Is Dissolved in Solution."
Post a Comment